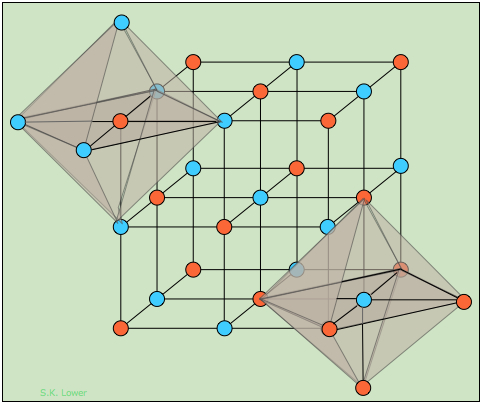
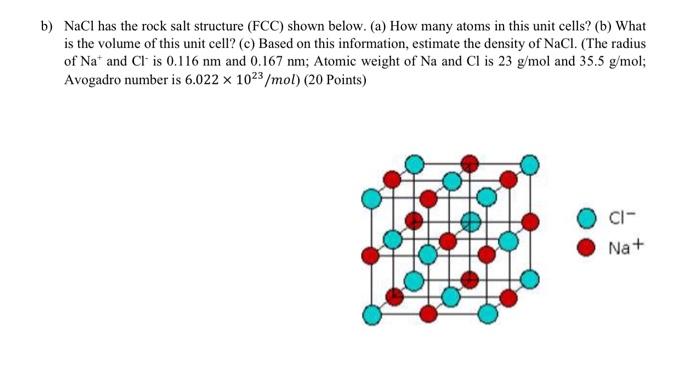
In a solid having rock salt structure, if all tge atoms touching one body diagonal plane are removed (except at body centre), then the formula of the - Sarthaks eConnect | Largest

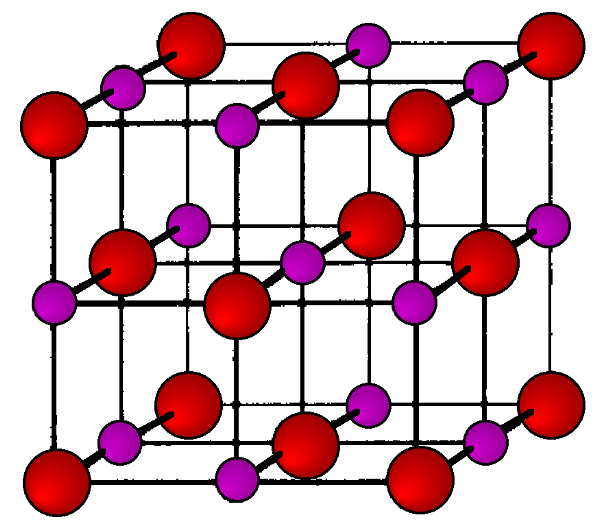
Sodium Chloride (rock Salt, Halite, Table Salt), Crystal Structure Stock Illustration - Illustration of road, atoms: 187037792

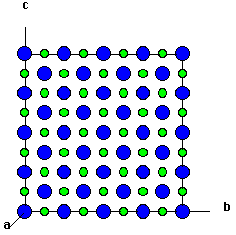
Face-centered cubic structure of rocksalt SnTe. The unit cell (green... | Download Scientific Diagram
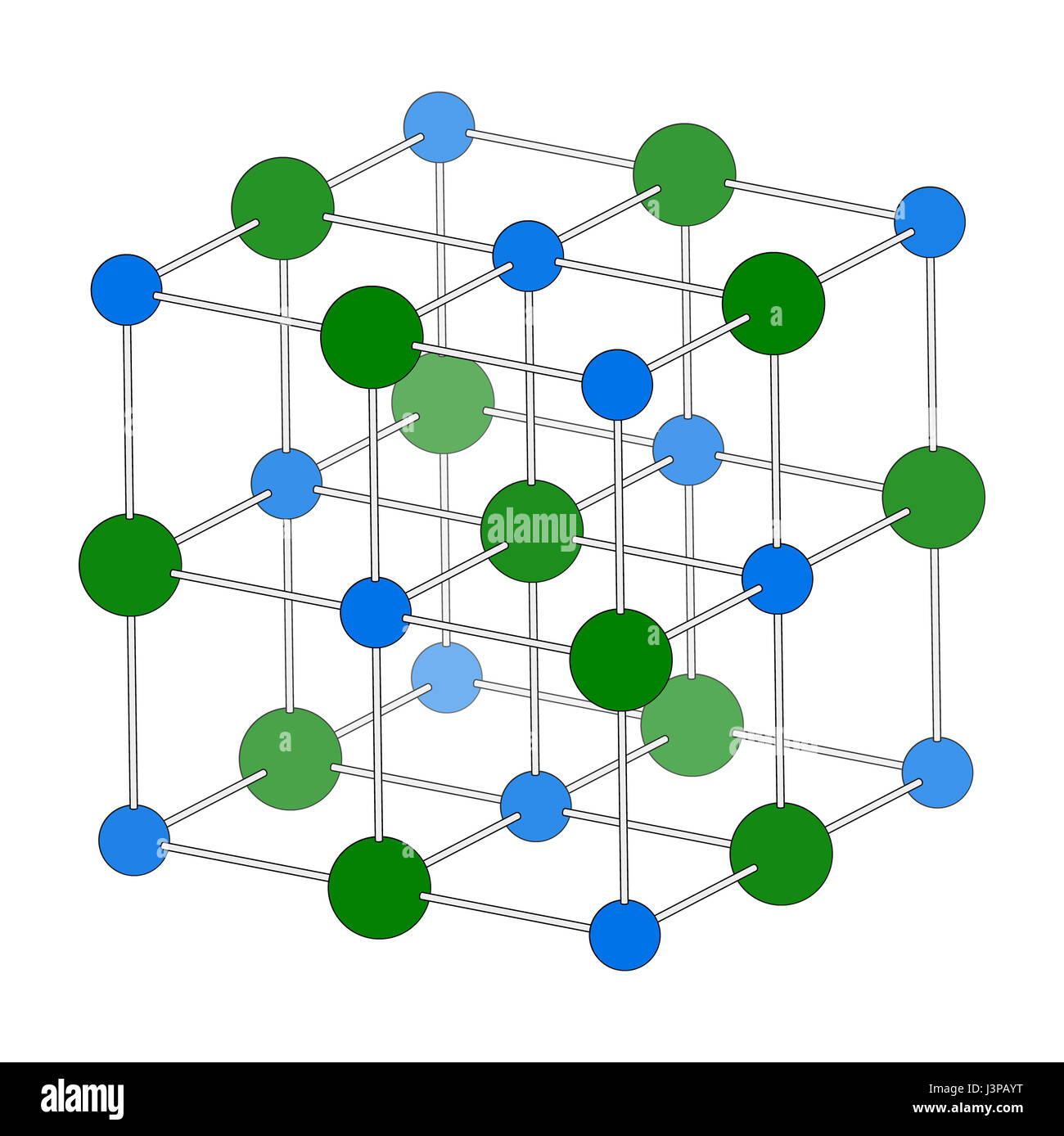
Sodium chloride (rock salt, halite, table salt), crystal structure. Atoms shown as color-coded spheres (Na, blue; Cl, green). Unit cell Stock Photo - Alamy

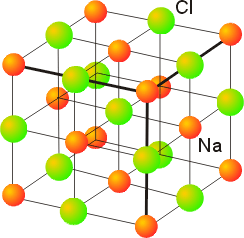
Unit cell representation for the rock salt structures of (A) AgCl and... | Download Scientific Diagram

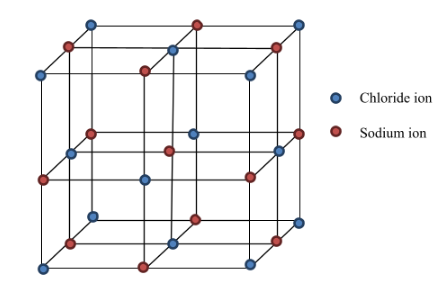
Rock salt structure,sodium chloride structure, ionic compound, ionic crystal type | By AJIT KANSHIDE - YouTube