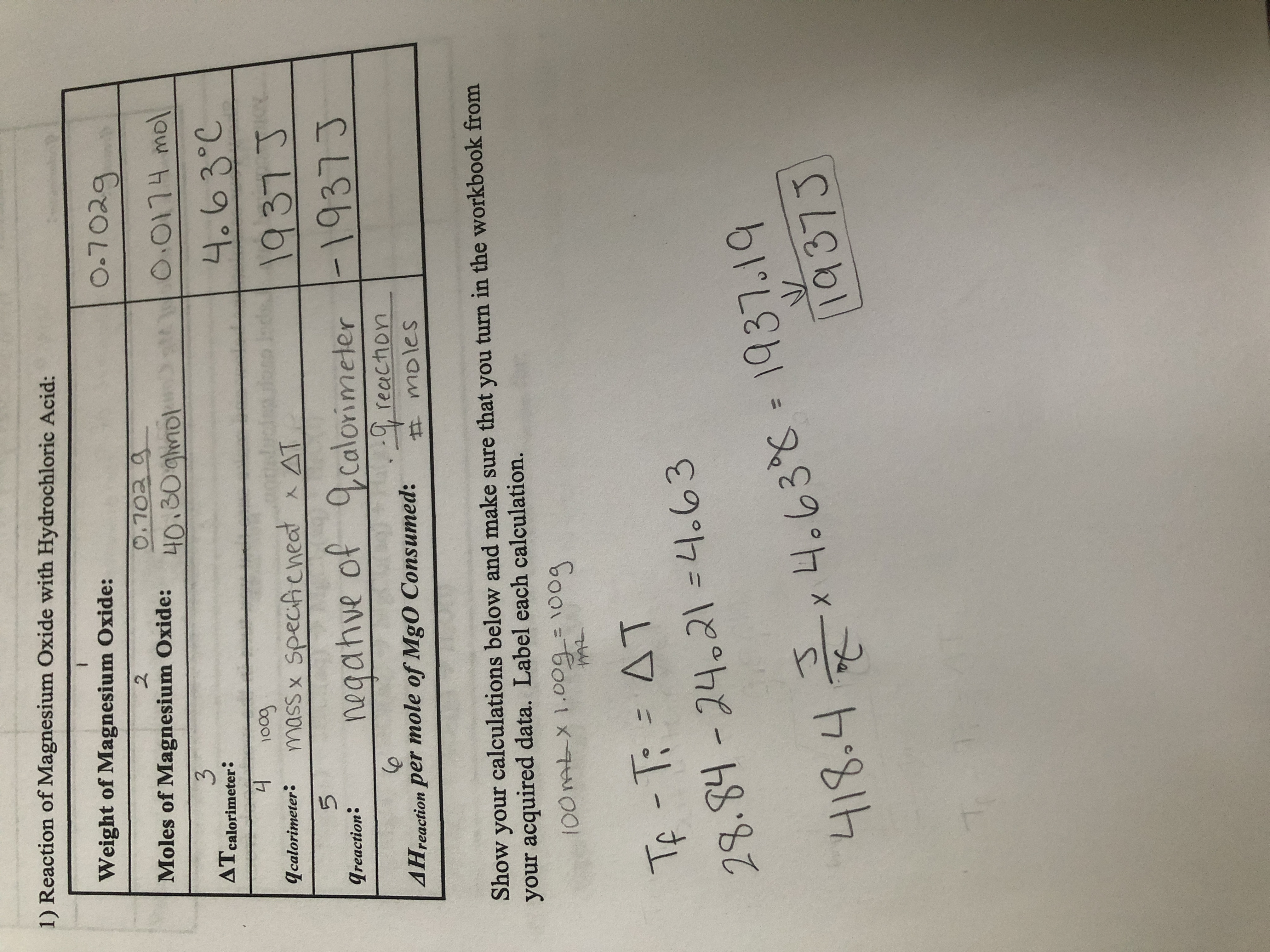
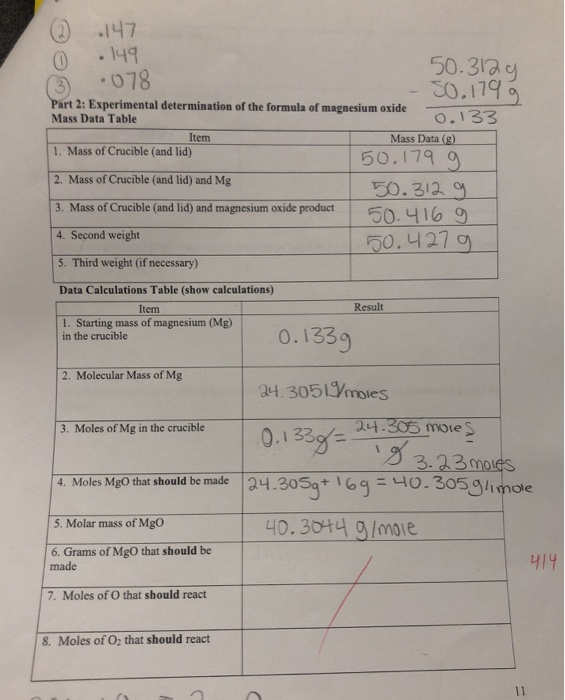
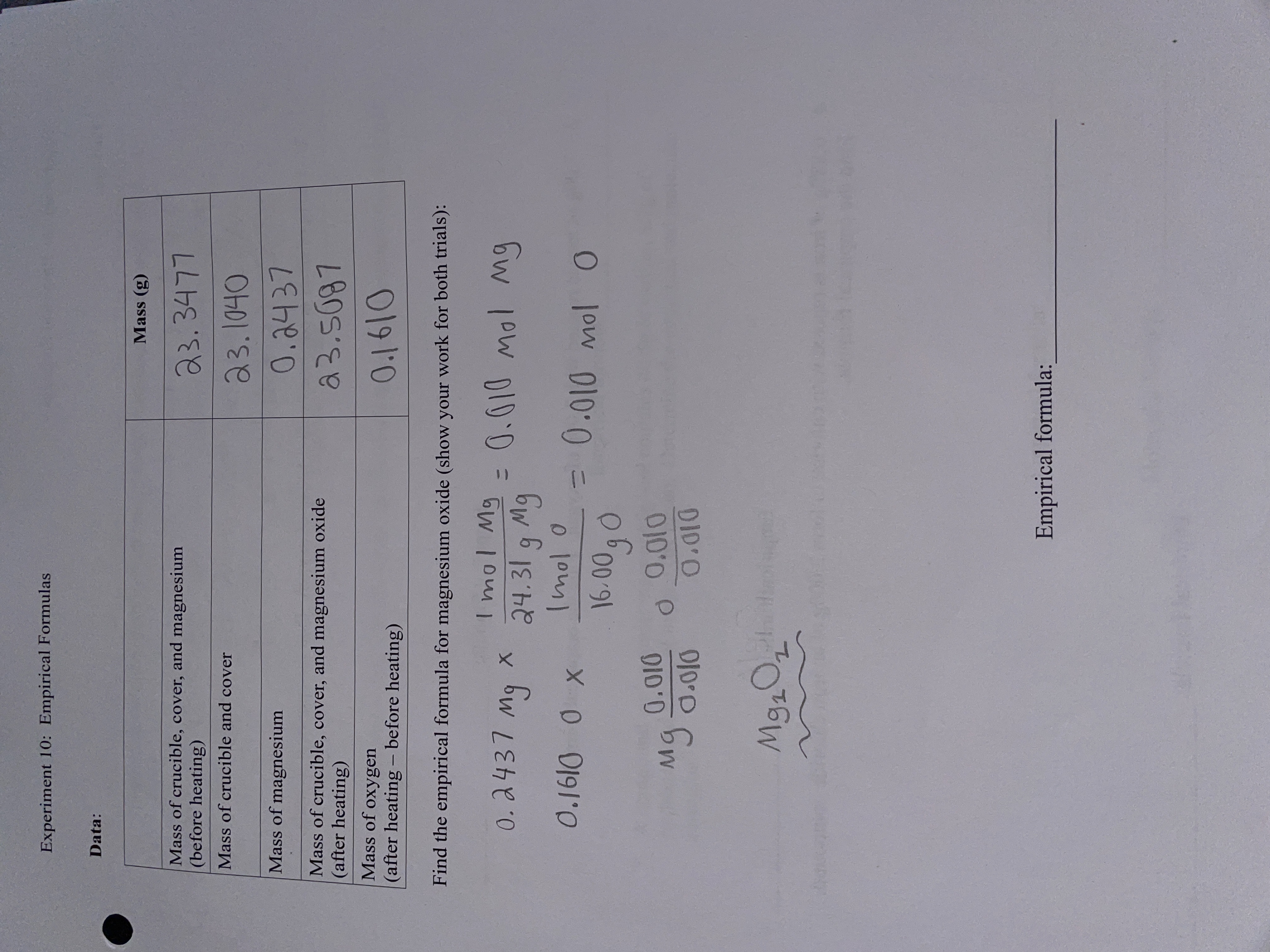
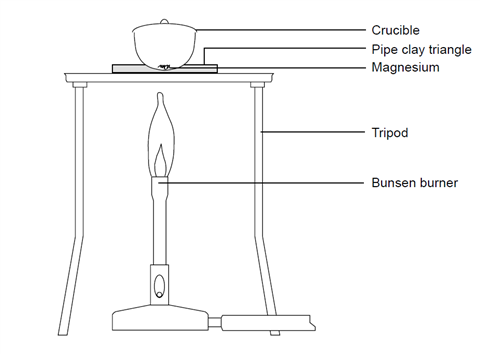
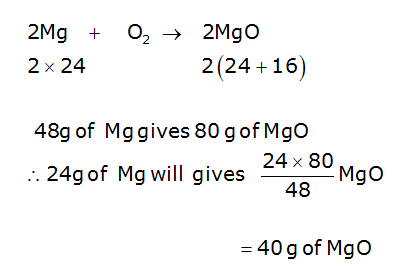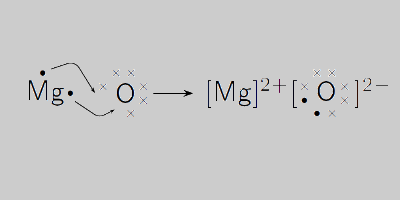A 3.250g sample of magnesium is burned in a container of 12.500g oxygen. What mass of oxygen gas remains unreacted after the magnesium has been completely consumed to form magnesium oxide as
![Calculate the number of molecules present in 0.5 moles of magnesium oxide ( MgO) . [Atomic weights : Mg = 24, O = 16 ] Calculate the number of molecules present in 0.5 moles of magnesium oxide ( MgO) . [Atomic weights : Mg = 24, O = 16 ]](https://dwes9vv9u0550.cloudfront.net/images/9085118/105391b5-2617-461a-9970-1a42367b289a.jpg)
Calculate the number of molecules present in 0.5 moles of magnesium oxide ( MgO) . [Atomic weights : Mg = 24, O = 16 ]

N. Calculate the number of moles ofmagnesium oxide, MgO in i. 80 g andii. 10 g of the compound. - Brainly.in
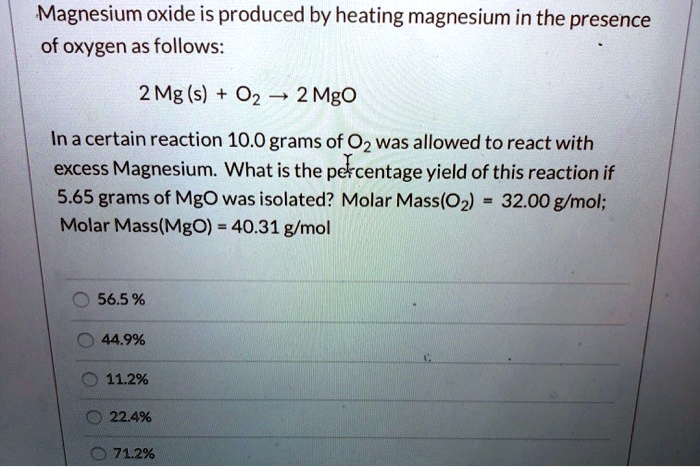
SOLVED: Magnesium oxide is produced by heating magnesium in the presence of oxygen as follows: 2 Mg (s) 02 2 Mgo In a certain reaction 10.0 grams of 0z was allowed to

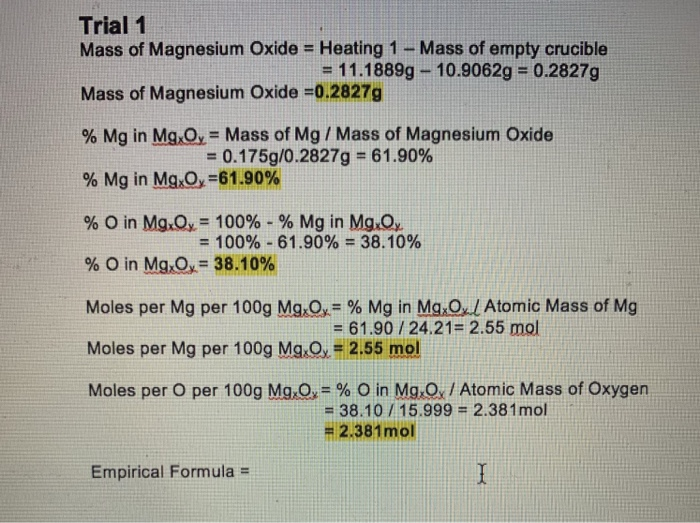
PDF) Experimentally Determining of the Empirical Formula of Magnesium Oxide | Folk Narongrit - Academia.edu

DOC) IB Chemistry IA: Determining the Empirical Formula of Magnesium Oxide | Josephine Yeh - Academia.edu