SOLVED:A 6.53 -g sample of a mixture of magnesium carbonate and calcium carbonate is treated with excess hydrochloric acid. The resulting reaction produces 1.72 L of carbon dioxide gas at 28^∘ C

For Your Research. The Four Research Questions 1.What is the chemistry (including an equation) of the process? 2.What are the factors that impact on the. - ppt download

SOLVED: 6(20 Rts) ^ mixture containing Magnesium Carbonate (MgcOxs) is reacted with an excess of hydrochloric acid according to the following equation: MgCO3) 2HCltaa) MgClzea) + CO(g) HzOqu) Calculate the " percentage

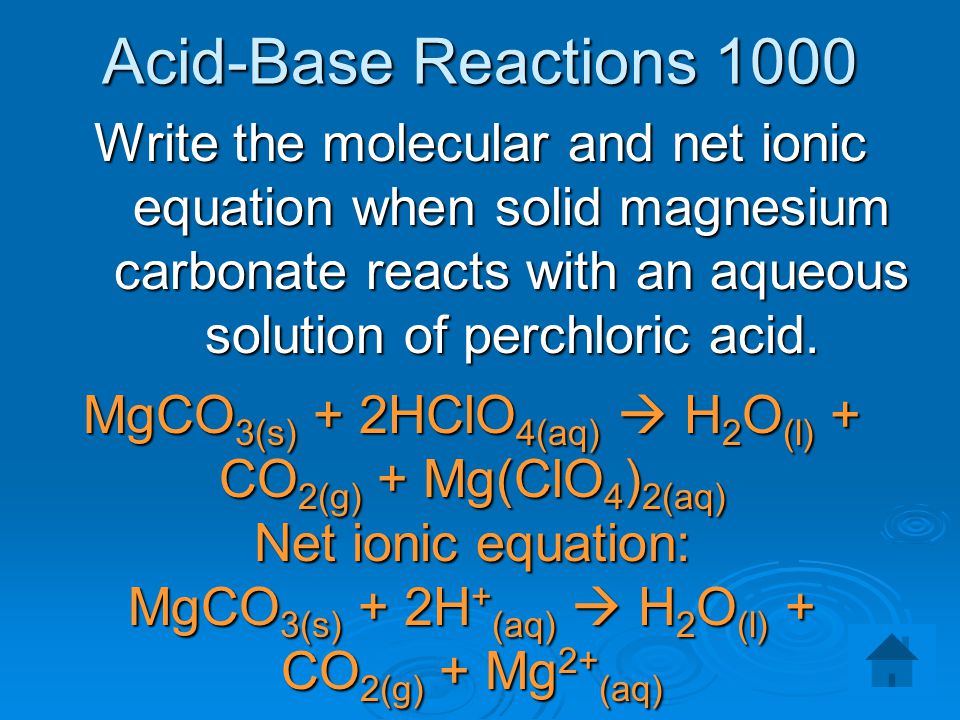
SOLVED:Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a net ionic equation for the reaction that occurs
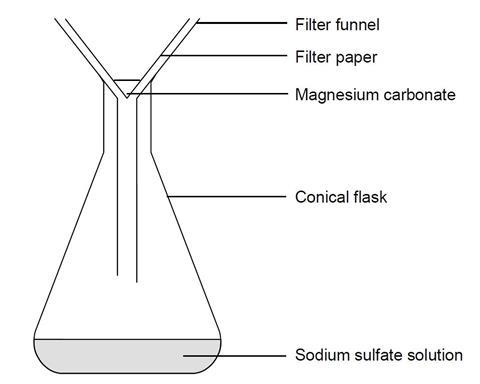
Making magnesium carbonate: the formation of an insoluble salt in water | Experiment | RSC Education